The unpaired electron when it comes near the pair of electrons can t help but swipe one of them to pair with itself.
Wyh does free radicalization of vinyl monomers.
The carbon carbon double bond in a vinyl monomer like ethylene has a pair of electrons which is very easily attacked by the free radical.
Free radical polymerization of vinyl monomers.
The reaction ends when either all.
Free radicals can be formed by a number of different mechanisms usually involving separate initiator molecules.
Vinyl molecules have a double bond of the type ch 2 chx and ch 2 cxy where x and y represent a number of possible chemical moieties such as a hydrogen atom ethylene a methyl group propylene or.
Monomer molecules and free radical initiators are added to a water based emulsion bath along with soaplike materials known as surfactants or surface acting agents.
The relatively non specific nature of the free radicals towards vinyl and other unsaturated monomers makes frp one of the most versatile polymerization methods.
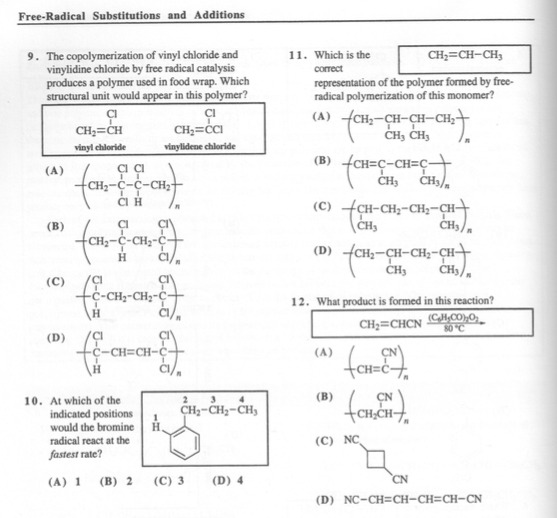
Compared to other fluoropolymers like polytetrafluoroethylene teflon pvdf has a low.
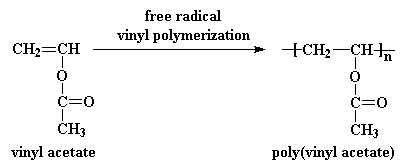
Vinyl acetate ch 2 cho 2 cch 3 is prepared from ethylene by reaction with oxygen and acetic acid over a palladium catalyst under the action of free radical initiators vinyl acetate monomers single unit molecules can be linked into long branched polymers large multiple unit molecules in which the structure of the vinyl acetate repeating units is.
Polyvinylidene fluoride or polyvinylidene difluoride pvdf is a highly non reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride.
These radicals react with the in the water dissolved monomers and form soap type free radicals.
Pvdf is a specialty plastic used in applications requiring the highest purity as well as resistance to solvents acids and hydrocarbons.
Emulsion polymerization is one of the most important methods for the polymerization of a large number of monomers like vinyl acetate vinyl chloride chloroprene acrylamide acrylates and methacrylates.
The surfactant molecules composed of a hydrophilic water attracting and hydrophobic water repelling end form a stabilizing emulsion before polymerization by coating the.
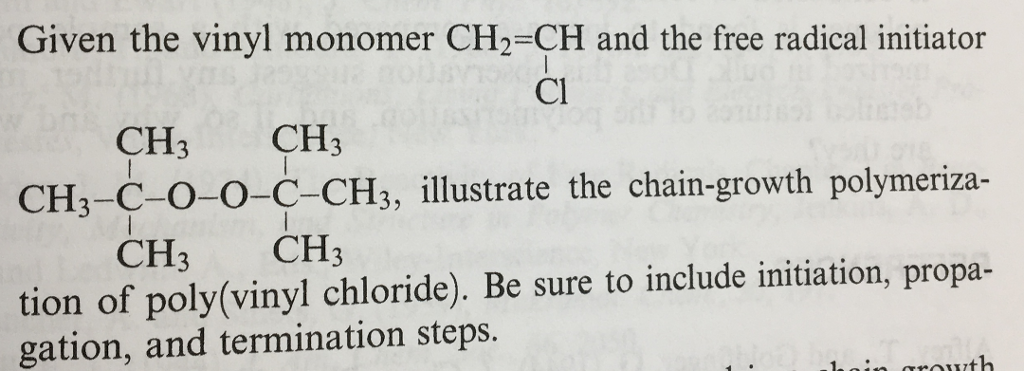
Free radical polymerization frp is one of the most important synthesis routes for obtaining vinyl polymers.
Free radical polymerization frp is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks.
One type of compound added as a stabilizer to vinyl monomers is an alkylated phenol 10 which can transfer its phenolic hydrogen to form a new radical eq.